What is Sodium Carbonate ?
Sodium carbonate stands for Na2CO3. This sodium carbonate is known by many names. Laundry soda is also known as gray soda and the soda crystals known to most people.
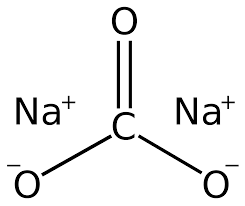
Sodium Carbonate
- Molar Mass 105.9888 g/mol
- Density 2.54 g/cm³
- Melting point 851 °C
- Boiling point 1,600 °C
- PH – 7

Sodium Carbonate Balancing Equation:
Ex : 1 Sodium carbonate also emits carbon-dioxide gas when it is heated by any corbanate salt.
Na2CO3 = Na2O + CO2
Ex : 2 Sodium carbonate also forms a salt called sodium chloride when hydrochloric acid is added. With it comes out water and carbon dioxide.
Na2CO3 + HCl → NaCl + CO2 + H2O
Sodium Carbonate Uses:
- Sodium carbonate is widely used to remove paper, glass, rayon fiber and dirt.
2. Sodium carbonate is used extensively in the preparation of soft drinks and soft drinks.