Basics of Green Chemistry:
Green chemistry is a chemical philosophy encouraging the design of desired products and eliminate the generation of hazardous substances. It mainly focuses on the desired yield with eco-friendly byproducts.
The aim of Green Chemistry is to start the reaction with cheaper starting material and environmentally safer one also.
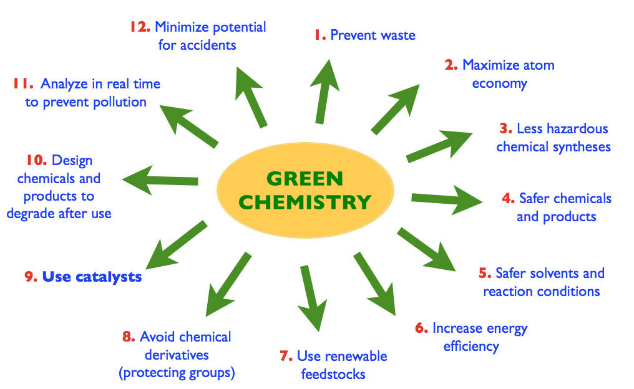
According to the work carried out by Paul T. Anastas, the following principles of green chemistry has been formulated,
- Select appropriate starting materials.
- Designing of safer chemicals.
- Select appropriate solvent.
- Use of catalysts should be preferred.
- Use of protecting groups should be avoided.
- Energy requirement should be minimum.
- Maximum conversion of starting materials and reagents into final products.
- Prevention of waste byproducts.
- Prevention of hazardous products.
- Products obtained should be bio degradable.
- Strengthening of analytical techniques to control hazardous compounds.
- The manufacturing plants should be designed to eliminate the possibility of accidents.
Atom Economy:
Atom economy is defined as the measure of the amount of starting material which remains at the end of the reaction.
Atom Economy = molecular wt of desired product/ molecular wt of all products *100 %
Reaction Yield:
Reaction yield is defined as the amount of pure and dry products which obtained in the reaction.
Reaction Yield = quantity of product isolated / theoretical quantity of product * 100%
Green Chemistry in day to day life:
- Dry cleaning of clothes:
For dry cleaning of clothes, solvents like tetrachloroethylene is used which is carcinogenic in nature and pollute the ground water also. Instead of that liquefied CO2 can be used. It is not harmful to the ground water. Now a days H2O2 can be used for better result and utilizes le s water.
2. Bleaching of paper.
Conventional method of bleaching was done with chlorine. Now a days eco-friendly H2O2 with catalyst can be used for bleaching of paper.
3. Synthesis of chemicals.
Many chemicals have been prepared with maximum yield.
4.Instead of petrol, methanol is used as a fuel in automobiles.
5. Neem based pesticides have been synthesized, which are more safer than the chlorinated hydrocarbons.