Lead Nitrate Definitions :
Lead nitrate is an inorganic compound. Lead nitrate is usually found as colorless or whitish particles. Unlike other chemical salts it is soluble in water.
- Molecular Formula : Pb(NO₃)₂
- Molecular Weight : 331.2 g/mol
- IUPAC Name : Lead(II) nitrate
- Boiling point : 83 °C
- Soluble in : Water

Commercial production of lead nitrate began in Europe and the United States in the 19th century. This compound is mainly used as a raw material in the manufacture of pigments and dyes. After the introduction of titanium dioxide, the lead nitrate was pushed back. Due to industry nylons and polyesters are used as stabilizing and thermodynamic coatings. Lead nitrate acts as a toxic oxidizer. This lead nitrate is used by the International Agency for Research on Cancer. Dissolve this compound in nitric acid and separate the precipitate.
Other Names :
- Carbonic Nitrate
- Nitrate of plumbum
- Carbonic dinitrate
- Plumb Dulcisu
Lead Nitrate Formula :
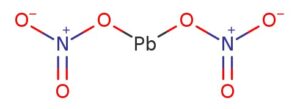
Let’s add (NO3) 2 times to make lead and di valent cation (NO3) univalent anion charge balance. After that it is called lead nitrate.
Chemical Reactions:
After heating lead nitrate we get lead oxide and nitrogen dioxide along with oxygen. An oxygen reacts with lead to form lead oxide. The remaining two react with oxygen and nitrogen to form nitrogen dioxide.
Lead Nitrate Uses :
1. Lead nitrate is used in special explosives and match industry carbonic acid as it has very fast burning property.
2. They were used in dyeing and printing of colored goods like calico.
Lead Nitrate Disadvantages :
1. Lead nitrate causes lung, brain, stomach and kidney cancer in humans.
2. Causes cancer in animals unlike humans.
3. Our high consumption of lead can cause anemia, weakness and brain damage.
4. It affects the nervous system of developing children.