What is Calcium Carbonate ?
CaCO3 means calcium carbonate. Calcium carbonate is a mineral commonly found in rocks but is also known as calcite and aragonite i.e. limestone.
Molar mass 100.0869 g/mol
Density 2.71 g/cm³
Melting point 825 °C
Equivalent Weight 50.045
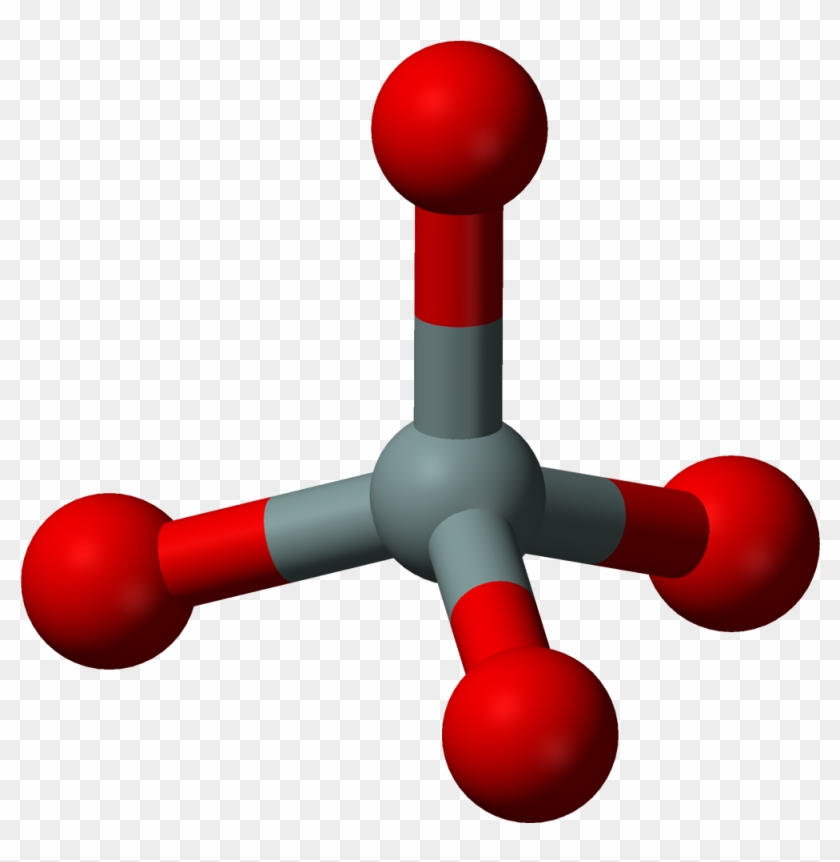
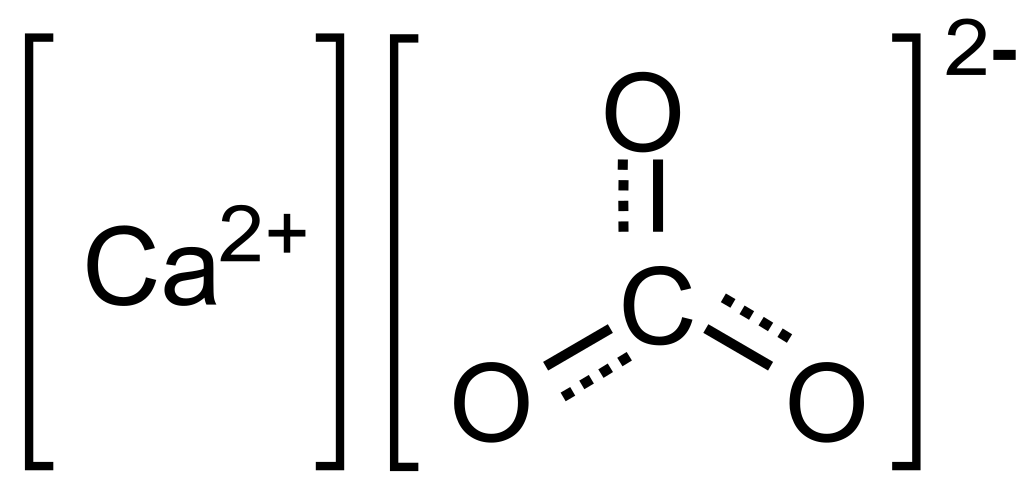
Calicium Carbonate Structure:
Calcium Carbonate Balancing Equation:
Example 1
Carbon dioxide gas evloution occurs when calcium carbonate is heated. The remaining oxygen gas combines with calcium to form calcium oxide. We can also commonly refer to this as metal oxide.
CaCO3 → CaO + CO2
Example 2
Calcium carbonate and H2O are available when calcium hydroxide reacts with carbon dioxide.
READ What is Fourier Transform Infra Red Spectroscopy, Introduction ,Principle, Advantages,Factors ?
Ca (OH)2 + CO2 → CaCO3 + H2O
Example 3
When calcium chloride reacts with sodium carbonate we get calcium carbonate and sodium chloride during double decomposition.
CaCl2 + Na2CO3 → CaCO3 + 2NaCl
Calcium Carbonate Uses:
- Calcium carbon is also used in medicine.
- Used to make toothpaste and calcium oxide.
- Calcium carbonate is used in agriculture to reduce soil acidity. Not only that but it also raises the pH value in the soil.